Training
Best Practices for YCANTH administration
Please review all 10 steps of the YCANTH Instructions for Use (IFU).
DOWNLOAD YCANTH IFUThe IFU must always be followed as the primary resource for guidance on use of the YCANTH applicator. Below are additional best practices for preparing the YCANTH applicator for treatment.
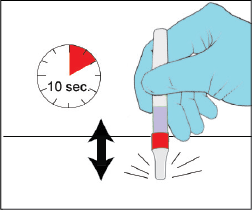
Gently Tap the YCANTH applicator to move the YCANTH solution
(Step 6 of IFU)
Gently tap the capped end of the YCANTH applicator on a horizontal surface for approximately 10 seconds or until the YCANTH solution has collected at the bottom of the applicator tube.
Best-practice tips
- Only tap if YCANTH solution has not collected at the bottom of the applicator tube
- Gravity moves the solution to the tip of the applicator
- Do NOT apply pressure to the applicator tube during this step
- Do NOT push the drug solution through the filter during this step
- Invert the applicator (seesaw motion) after EACH lesion is treated to avoid YCANTH on healthy skin
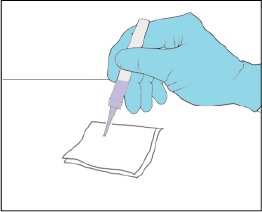
Test the YCANTH applicator (Step 7 of IFU)
Remove the applicator cap. Gently squeeze the applicator tube to apply a droplet to a paper towel or gauze to confirm the YCANTH applicator is working properly.
Best-practice tips
- Establish the flow of YCANTH solution before dispensing onto a molluscum lesion. The key here is to establish the amount of solution and flow based on finger pressure and angle of the applicator when testing onto the gauze BEFORE the solution is delivered to a lesion
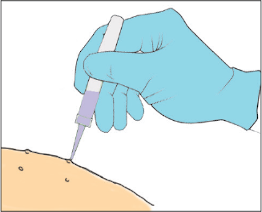
Apply a small droplet of the YCANTH solution onto a molluscum lesion (Step 8 of IFU)
Use the applicator tip to spread the solution to cover the entire lesion.
Best-practice tips
- Most molluscum lesions are 1 to 5 mm in diameter. The tip of the applicator is 1 mm in diameter and designed to deliver a targeted dose of YCANTH to each lesion. You need very little drug to cover an entire molluscum lesion with a thin film of drug solution. There is no need to spread YCANTH if the lesion is only 1 to 3 mm because that is about the size of a small droplet
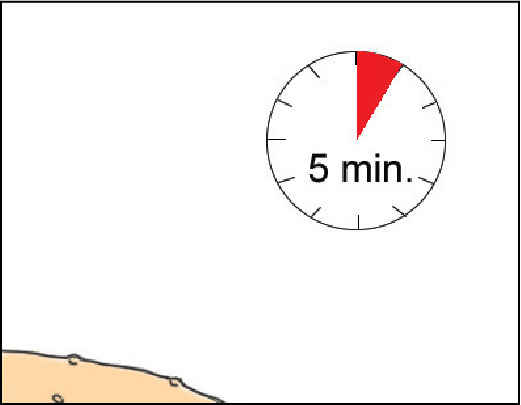
Allow YCANTH Solution to completely dry, up to 5 minutes. (Step 9 of IFU)
This keeps YCANTH off healthy skin.
Best-practice tips
- Instruct patient to be still and keep body horizontal while solution is drying.
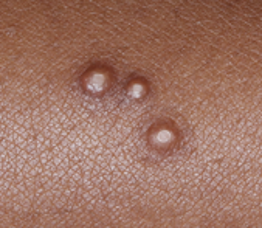
Clustered Lesions and/or Sensitive Skin Areas
Some patients will present with several small, clustered molluscum lesions, and/or lesions in sensitive skin areas, such as the face and armpit.
Best-practice tips
- In these cases, consider treating select but not all lesions. This will help to prevent multiple blisters coalescing into larger blisters.
- In the YCANTH clinical trials, all baseline and new treatable lesions were treated. For the first few times treating a patient with YCANTH, consider treating just one or two body regions, or select lesions.
Post Treatment Care
Instruct patients or caregivers to wash the treated skin area with soap and water 24 hours after treatment. Patients may wash YCANTH off skin sooner if they develop severe blistering, severe pain, or other severe reactions.
Best-practice tips
- Remind patient/caregiver that blisters and local skin reactions are expected. Blistering is a sign that YCANTH is working to help clear the bumps from the skin.
- Remind patients or caregivers to not use a washcloth, abrasive material, or vigorous rubbing because this could be painful.
- Over-the-counter pain relief medicines can be used as needed.
- Send patient home with YCANTH Post Treatment Care Instructions
Before administering YCANTH, please review the full Prescribing Information.
DOWNLOAD YCANTH PI

