About
TREAT NOW WITH THE FIRST FDA-APPROVED THERAPY FOR MOLLUSCUM
FOR AGES
2+
YCANTH®—precise control with proven results1
YCANTH—designed to ensure a consistent treatment experience with a controlled formulation in each applicator1
The innovative applicator contains an ether-free formulation of cantharidin within a sealed glass ampule. YCANTH must be administered by a trained, licensed healthcare professional.2
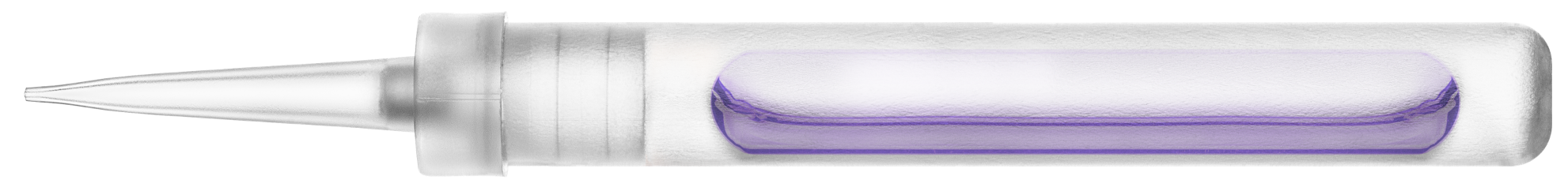
- Small applicator opening allows for targeting of each lesion
- A coloring agent helps prevent multiple applications to the same lesion
- GMP-controlled, consistent formulation of 0.7% (w/v) cantharidin
- Single-use applicator
- Prepared by breaking the glass ampule with a YCANTH Break Tool to release YCANTH into the applicator
- Contains a bittering agent to help deter ingestion

- Small applicator opening allows for targeting of each lesion
- A coloring agent helps prevent multiple applications to the same lesion
- GMP-controlled, consistent formulation of 0.7% (w/v) cantharidin
- Single-use applicator
- Prepared by breaking the glass ampule with a YCANTH Break Tool to release YCANTH into the applicator
- Contains a bittering agent to help deter ingestion
Applicator is not to scale.
YCANTH is the only FDA-approved formulation of cantharidin2
The FDA warns that compounded copies of an approved product will unnecessarily expose a patient to drug products that have not been shown to be safe and effective. See the FDA guidance here.
Learn how to apply YCANTH for your patients by watching a step-by-step instructional video.
WATCH VIDEOYCANTH is easily applied in the office
Using the small-tip applicator, apply only enough to cover each lesion2*
- YCANTH may be administered every 3 weeks, as needed2
- If YCANTH comes in contact with healthy skin during application, it should be removed with a cotton swab or gauze2
- Avoid application near the eyes and mucosal tissues, and to adjacent healthy skin2
YCANTH should be removed with soap and water 24 hours after treatment2
- Do not cover treated lesions with bandages2
- If severe blistering, severe pain, or other adverse reactions occur, remove YCANTH prior to the recommended 24 hours after administration2
What you should know before administering YCANTH
- Verrica is committed to providing a training program to ensure an optimal experience
- Counsel your patients to avoid contact with the treatment areas, including oral contact2
- YCANTH is flammable; avoid fire, flame, or smoking near lesion(s) during treatment2
- YCANTH is not for oral, mucosal, or ophthalmic use2
*
YCANTH should be applied only to lesions that are practically and medically safe to treat.2
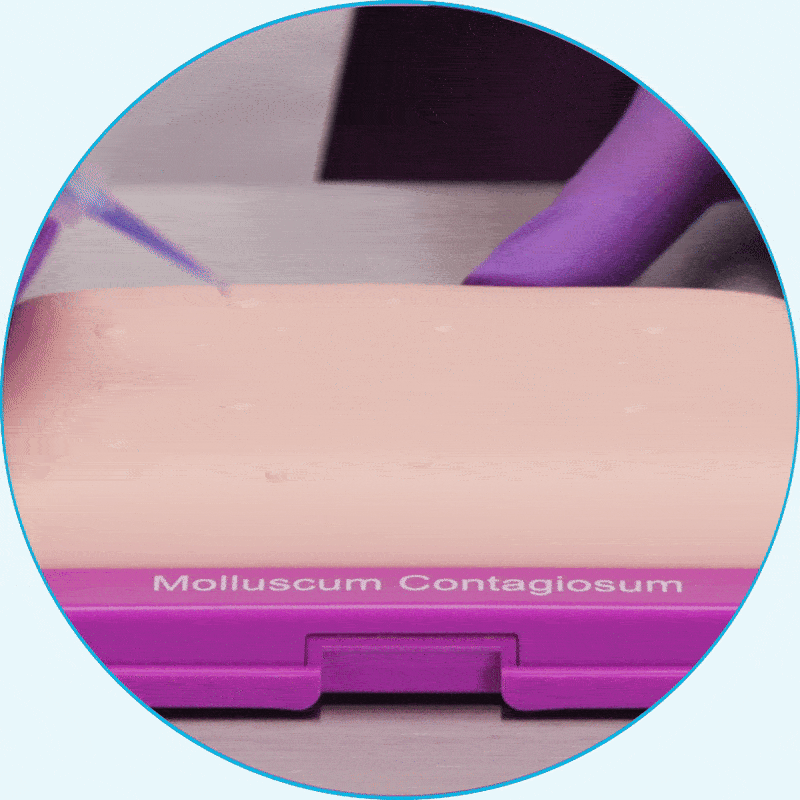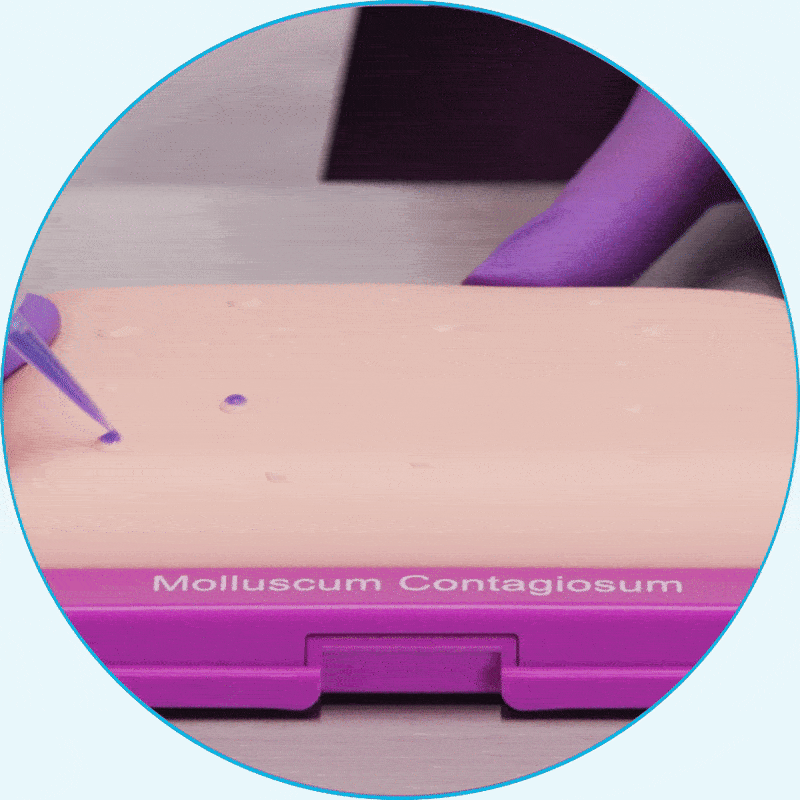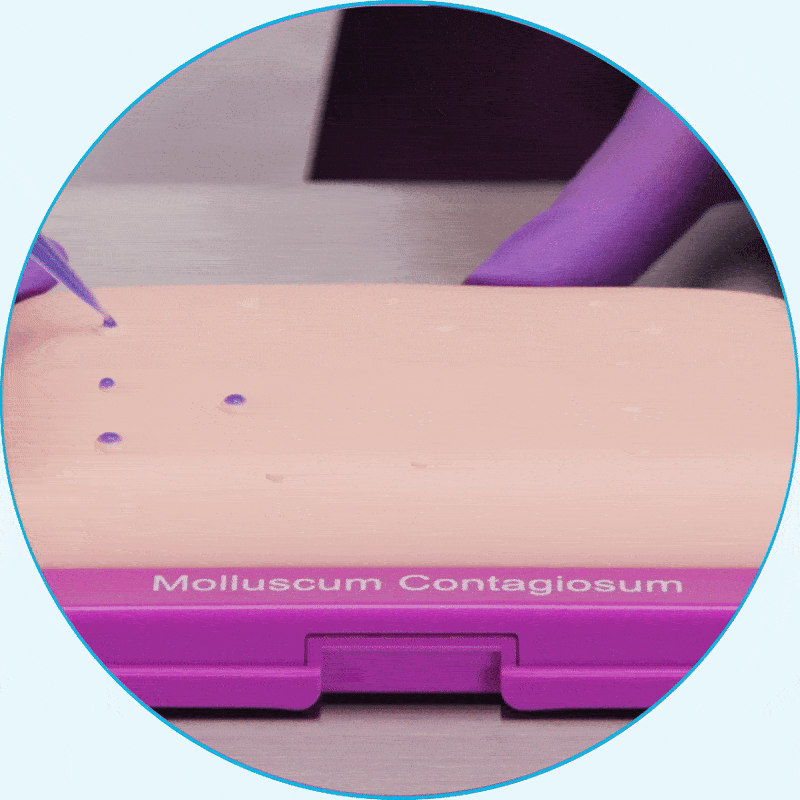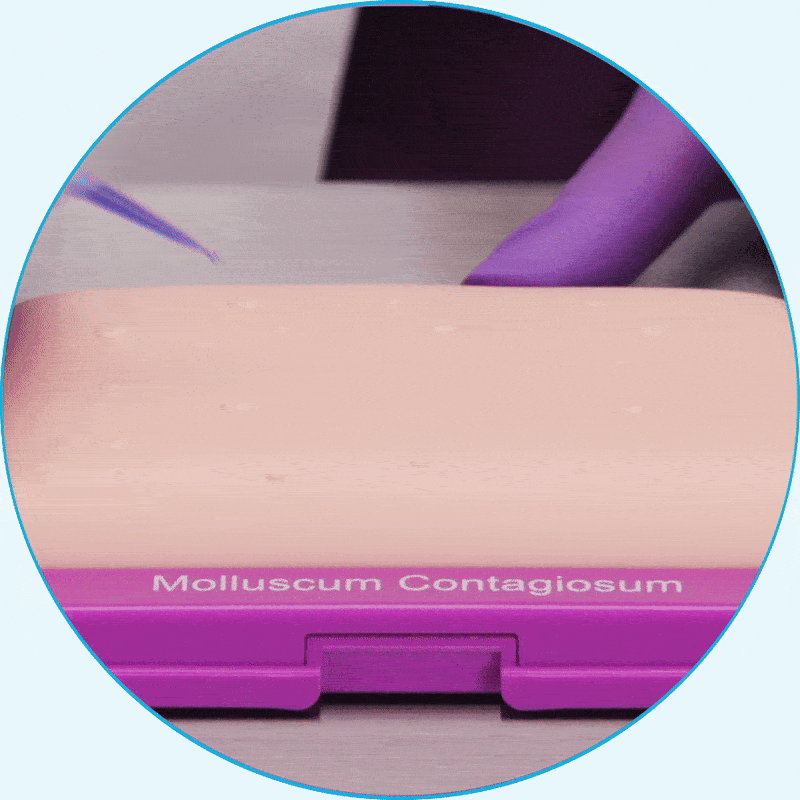
Hear from clinical experts about the ease of use of YCANTH
The opinions expressed within the video content are solely the speaker's and do not reflect the opinions and beliefs of Verrica Pharmaceuticals.
Consistent formulation, precise control.1
In two phase 3 clinical trials, significantly more patients treated with YCANTH achieved complete clearance of new and baseline lesions compared with vehicle within 12 weeks (P<0.0001)1,3
YCANTH is a vesicant. Local skin reactions at the application site were observed in 97% of subjects treated with YCANTH. In clinical trials, no serious adverse reactions were reported.2

