YCANTH has strong insurance coverage across commercial and Medicaid plans
- 90% coverage across the top commercial plans1†
- Covered by many state Medicaid and managed Medicaid plans
†Based a review of the top 50 health plans in the United States.1
Verrica Pharmaceuticals offers a flat-rate copay program for each YCANTH treatment visit. The program simplifies the patient’s out-of-pocket cost determination for all commercially insured patients.*
Patients with commercial insurance can pay just $25 per YCANTH treatment visit with the Program:*
Offices using Y-Access® Support Solutions or Verrica's Specialty Pharmacy Network can have their patient automatically enrolled into the Copay Assistance Program.
Per treatment visit, up to two applicators:
Remind patients that flexible spending accounts (FSA) or health savings accounts (HSA) can help cover copay expenses. Healthcare expense accounts can help offset qualified out-of-pocket expenses. Encourage patients to check with their program provider. Additional terms and conditions may apply.
TRICARE® is a registered trademark of the Department of Defense (DOD), DHA.
Visit Y‑AccessSupport.com to learn about the eligibility requirements and enroll your patients in the Copay Assistance Program
The list price, or Wholesale Acquisition Cost (WAC), is $685 per applicator of YCANTH as of July 2023. The WAC may not reflect the price paid by patients.
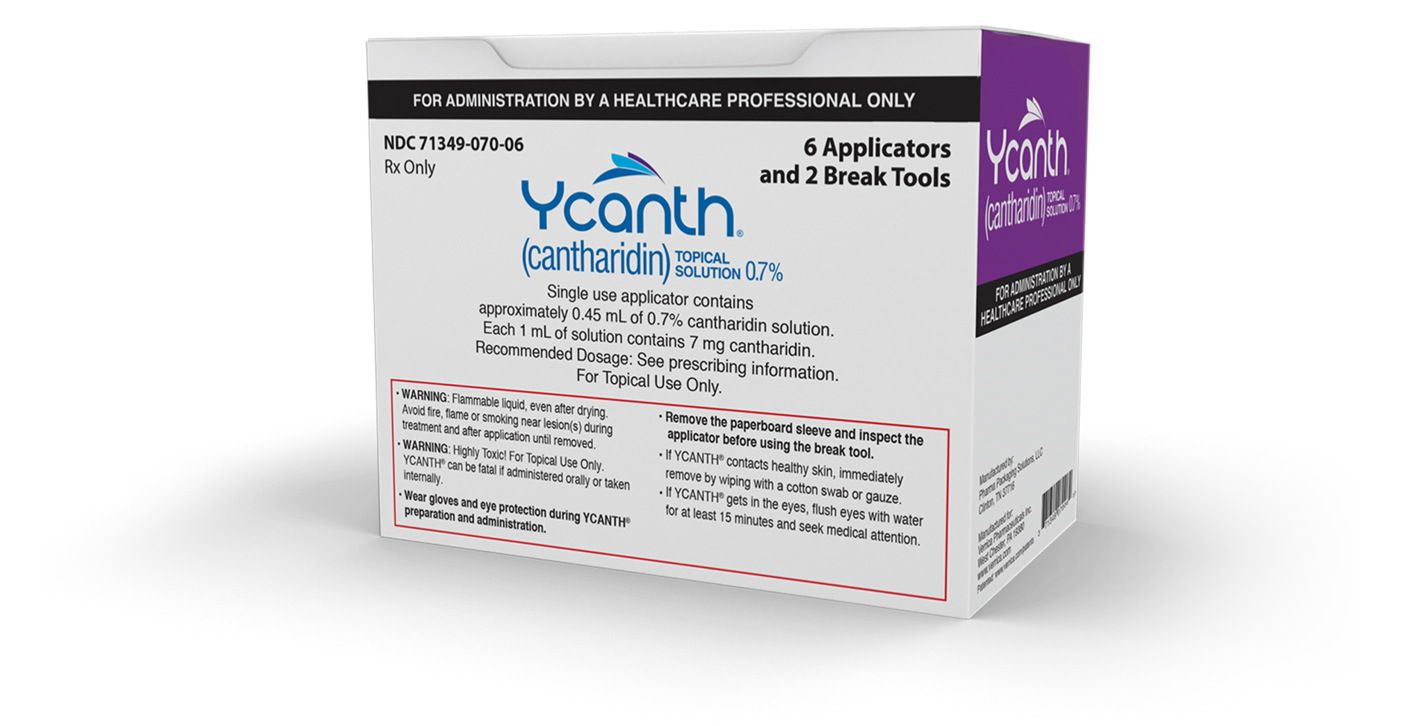
Photo is for illustrative purposes only.
Call 1-877-VERRICA (1-877-837-7422) to find out how much YCANTH may cost for your patients
YCANTH has strong insurance coverage across commercial and Medicaid plans
†Based a review of the top 50 health plans in the United States.1

Photo is illustrative and not representative of all patients.
Questions about insurance or coverage for your patients?
Contact a Field Reimbursement Manager (FRM) at marketaccess@verrica.com or call 1-877-VERRICA (1-877-837-7422)

Model is for illustrative purposes only.
For more helpful resources and additional information, visit YCANTHPro.com/office-resources.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS: None.
WARNINGS AND PRECAUTIONS:
INDICATION
YCANTH (cantharidin) topical solution, 0.7% is indicated for the topical treatment of molluscum contagiosum in adult and pediatric patients 2 years of age and older.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS: None.
WARNINGS AND PRECAUTIONS:
ADVERSE REACTIONS:
The most common (incidence ≥1%) reactions are the following local skin reactions at the application site: vesiculation, pain, pruritus, scabbing, erythema, discoloration, application site dryness, edema, and erosion. Local skin reactions at the application site were observed in 97% of subjects treated with YCANTH during clinical trials. These local skin reactions are expected and related to the anticipated blistering response of the skin to cantharidin.
DRUG INTERACTIONS:
No studies evaluating the drug interaction potential of cantharidin have been conducted.
USE IN SPECIFIC POPULATIONS:
Pregnancy: There are no available data with use of YCANTH in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Given that systemic exposure to cantharidin following topical administration is low, maternal use is not expected to result in fetal exposure to the drug.
Lactation: Avoid application of YCANTH topical solution to areas with increased risk for potential ingestion by or ocular exposure to the breastfeeding child.
OVERDOSAGE:
Oral ingestion of cantharidin has resulted in renal failure, blistering and severe damage to the gastrointestinal tract, coagulopathy, seizures, and flaccid paralysis.
Please see full Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact Verrica Pharmaceuticals Inc. at 1-877-VERRICA (1-877-837-7422), or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Local skin reactions are expected and should be reported if they are severe.
Reference: 1. Data on file. Verrica Pharmaceuticals Inc., 2024.
DHA=Defense Health Agency; DOD=Department of Defense; NDC=National Drug Code; VA=Veterans Affairs.